Treatment for RVO
Current RVO treatments manage only the symptomatology from subsequent pathologic changes since there is no treatment to reopen the blood vessels.1 Anti-vascular endothelial growth factor (anti-VEGF) agents are the first line of treatment for macular edema secondary to RVO.2 Aflibercept and ranibizumab are FDA-approved, and bevacizumab may also be used off-label as RVO and macular edema treatments.2 Read below for clinical studies on anti-VEGF therapy and more.
Ranibizumab
Ranibizumab is a humanized, recombinant, affinity-matured VEGF monoclonal antibody fragment, designed for intraocular use that binds to and neutralizes all isoforms of VEGF-A and their biologically active degradation products.3-6
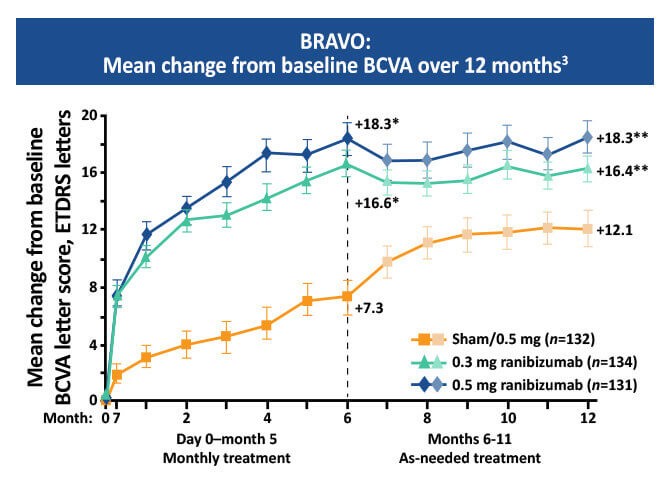
The BRAVO study showed that monthly injections of 0.3 mg or 0.5 mg ranibizumab resulted in a 16 to 18 letter gain in mean best corrected visual acuity (BVCA) compared to a 7.3 letter gain in the sham group at six months.3-5 This study also showed that delaying treatment can result in poorer outcomes.2
The COMRADE extension study showed ranibizumab had a better ocular safety profile and produced greater average BCVA compared to dexamethasone for treating macular edema in RVO patients.7 Additionally, the SCORES2 trial found that intravitreal bevacizumab was noninferior to aflibercept for treating macular edema due to CRVO at six months. 3,8,9
Aflibercept
Aflibercept is a receptor fusion protein of key domains from human VEGF receptors 1 and 2, with the constant region (Fc) of human immunoglobulin G that binds to multiple isoforms of VEGF-A, VEGF-B, and placental growth factor.3,10 Eyes treated with aflibercept showed a lower trend to progress to any neovascularization of the retina and iris as compared with the sham groups.3,11
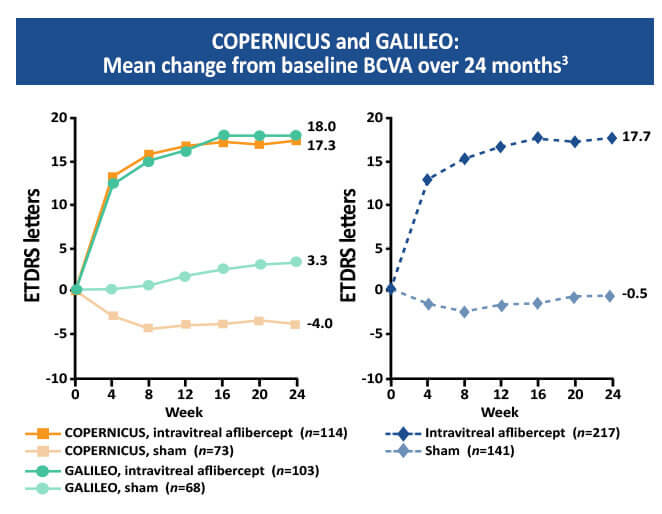
The Copernicus and Galileo studies were two parallel randomized double masked, phase 3 studies that every four weeks compared intravitreal 2mg aflibercept with sham treatment of macular edema secondary to CRVO.11 At week 24, mean BCVA change from baseline was +17.7 and -0.5 letters in the aflibercept and the sham group respectively.3,11
Corticosteroids
Steroids are considered a second-line therapy to treat inflammation associated with macular edema.12 Steroids inhibit the expression of the VEGF gene and the metabolic pathways of VEGF, and inflammatory cytokines all of which are involved in macular edema secondary to RVO.3,13,14
Steroid implants are more effective on visual gains in patients with macular vein occlusion versus branch retinal vein occlusions.12 The GENEVA study demonstrated that the biodegradable intravitreal implant containing 0.7 mg of dexamethasone resulted in improved visual acuity (VA), with a peak effect after 2 months (a mean gain of 10 letters) and a progressive decline to baseline values at 6 months.3
Steroid use is linked to increased risk of glaucoma and cataracts.15,16 Intravitreal steroids are an option for patients who don’t respond to anti-VEGF treatment; implants usually provide a lower treatment frequency compared to anti-VEGF.3,11
Panretinal laser photocoagulation (PRP)
Panretinal laser photocoagulation therapy is used to treat the complication of neovascularization associated with RVO by destroying the ischemic retina, reducing VEGF production and improving the blood supply to the rest of the retina.3,17 Argon laser is usually used for PRP, with the laser spots applied outside the vascular arcade extending anteriorly as far as possible.3
Focal laser photocoagulation
Focal laser photocoagulation to the macular region was the standard of care to treat macular edema secondary to BRVO before the advent of anti-VEGF drugs.3,18 The laser pulses in a grid pattern to help stop fluid leakage from the vessels.19 With recommended consideration as a second-line therapy, laser photocoagulation should be performed in eyes with macular edema for at least 3 months with a visual acuity of 20/40 or worse and without significant macular hemorrhage along with fluorescein angiography showing capillary perfusion in the absence of blood involving the fovea.3,15
References
- Cleveland Clinic. Retinal Vein Occlusion (RVO). Last reviewed July 17, 2019. https://my.clevelandclinic.org/health/diseases/14206-retinal-vein-occlusion-rvo.
- Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal Vein Occlusions Preferred Practice Patterns. Ophthalmology. 2019;127:P288-P320. https://pubmed.ncbi.nlm.nih.gov/31757503/
- Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, et al. Guideline for the Management of Retinal Vein Occlusion by the European Society of Retinal Specialists (EURETINA). Ophthalmologica. 2019;242:123-162. https://www.karger.com/Article/Pdf/502041
- Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: Six month primary end point results of a phase III study. Ophthalmology. 2010;117:1102-1112.e1. https://pubmed.ncbi.nlm.nih.gov/20398941/
- Brown DM, Campochiaro PA, Singh RP, et al: Ranibizumab for macular following central vein occlusion: Six-month primary endpoint results of a phase III study. Ophthalmology.2010;117:1124-1133.e1. https://pubmed.ncbi.nlm.nih.gov/20381871/
- Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: Implication of VEGF as critical stimulator. Mol Ther. 2008;16:791-799. https://pubmed.ncbi.nlm.nih.gov/18362932/
- Eter N. Efficacy and safety of ranibizumab 0.5mg versus dexamethasone 0.7mg in branch retinal vein occlusion: 6 month results of the COMRADE-B study. Invest Ophthalmol Vis Sci.2015;56:5806. https://iovs.arvojournals.org/article.aspx?articleid=2335878
- Scott IU, VanVeldhuisen PC, Ip MS, et al. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: The score2 randomized clinical trial. JAMA. 2017;317:2072–2087. https://pubmed.ncbi.nlm.nih.gov/28492910/
- Scott I. Macular edema associated with retinal vein occlusion. Retina Today. April, 2010:54-56. https://retinatoday.com/articles/2010-apr/macular-edema-associated-with-retinal-vein-occlusion
- Pielen A, Clark WL, Boyer DS, et al. Integrated results from the COPERNICUS and GALILEO studies. Clin Ophthalmol. 2017;11:1533–1540. https://pubmed.ncbi.nlm.nih.gov/28883712/
- Ashraf M, Souka AAR, Singh RP. Central retinal vein occlusion: Modifying current treatment protocols. Eye (Lond). 2016;30,505-514. https://pubmed.ncbi.nlm.nih.gov/26869163/
- Ashraf M, Souka A. Steroids in central vein occlusion: Is there a role in current treatment practice? J Ophthalmol. 2015;2015:594615. https://pubmed.ncbi.nlm.nih.gov/26635973/
- Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc Natl Acad Sci USA. 2008;105:4862–4867. https://pubmed.ncbi.nlm.nih.gov/18347336/
- Flynn HW Jr, Scott IU. Intravitreal triamcinolone acetonide for macular edema associated with diabetic retinopathy and venous occlusive disease: It’s time for clinical trials. Arch Ophthalmol. 2005;123:258–259. https://pubmed.ncbi.nlm.nih.gov/15710825/
- Morris R. Retinal vein occlusion. Kerala J Ophthalmol. 2016;28:4-13.
- Stuart A. Untangling retinal vein occlusion. Eyenet Magazine. November, 2013. https://www.aao.org/eyenet/article/untangling-retinal-vein-occlusion
- Stefánsson E. The therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiology. Acta Ophthalmol Scand. 2001;79:435–440. https://pubmed.ncbi.nlm.nih.gov/11594975/
- Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984;98:271–282. https://pubmed.ncbi.nlm.nih.gov/6383055/
- American Society of Retina Specialists (ASRS). Branch retinal vein occlusion. 2020. https://www.asrs.org/patients/retinal-diseases/24/branch-retinal-vein-occlusion