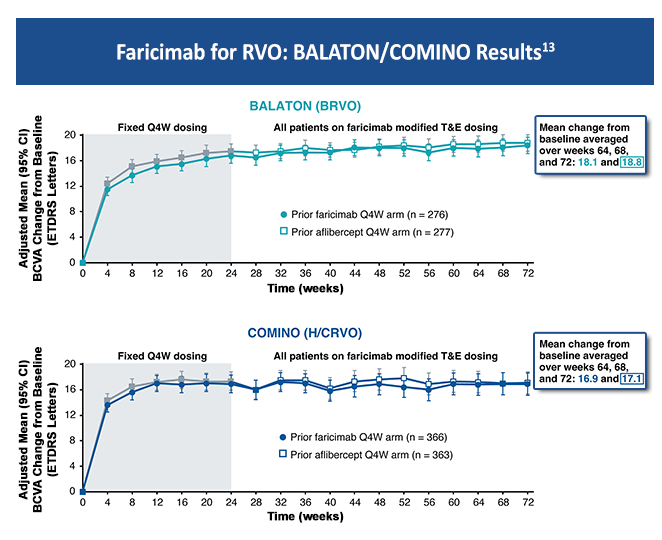
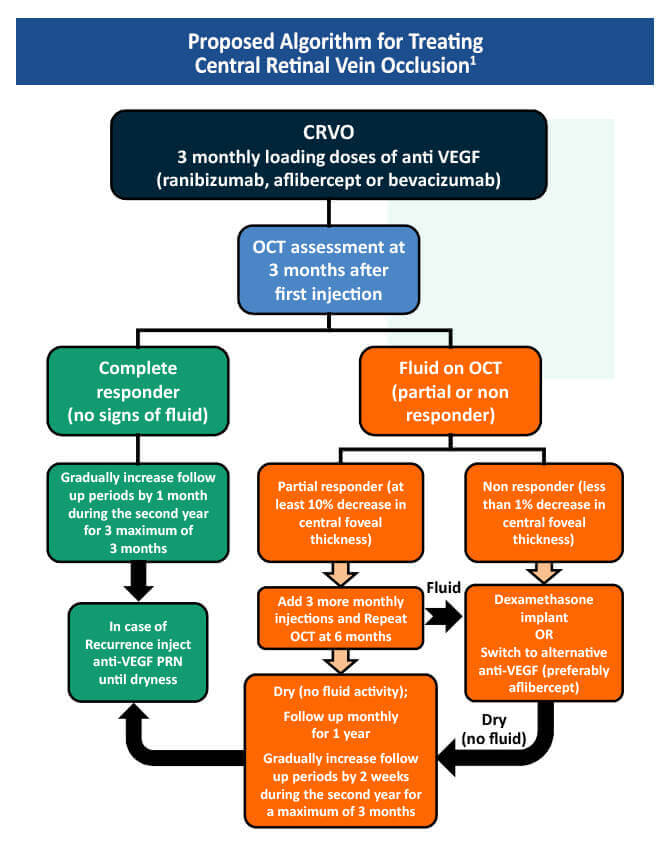
Scientific Council

Neil M. Bressler, MD
James P. Gills Professor of Ophthalmology
Professor of Ophthalmology, Johns Hopkins University School of Medicine
Wilmer Eye Institute, Johns Hopkins Medicine
Baltimore, MD

A. Paul Chous, MA, OD, FAAO
Specializing in Diabetes Eye Care & Education, Chous Eye Care Associates
Adjunct Professor of Optometry, Western University of Health Sciences
AOA Representative, National Diabetes Education Program
Tacoma, WA

Steven Ferrucci, OD, FAAO
Chief of Optometry, Sepulveda VA Medical Center
Professor, Southern California College of Optometry at Marshall B. Ketchum University
Sepulveda, CA

Julia A. Haller, MD
Ophthalmologist-in-Chief
Wills Eye Hospital
Philadelphia, PA

Allen C. Ho, MD, FACS
Director, Retina Research
Wills Eye Hospital
Professor and Chair of the Department of Ophthalmology
Thomas Jefferson University Hospitals
Philadelphia, PA

Charles C. Wykoff, MD, PhD
Director of Research, Retina Consultants of Houston
Associate Professor of Clinical Ophthalmology
Blanton Eye Institute & Houston Methodist Hospital
Houston, TX